Onsite wastewater Science and Technology
Technical article explaining the science behind wastewater
treatment.
Table of Contents
BASICS OF WASTEWATER TREATMENT
Before reading about the individual technologies discussed
later in this document, it is helpful to understand some of the basics
of wastewater treatment. You will see terms like BOD, total suspended solids,
nitrification, and denitrification frequently when discussing wastewater
treatment. It is important to understand what each of these terms mean
and how each relates to the wastewater treatment process. Some very basic
processes of wastewater treatment are also briefly discussed. If you understand
the theory behind these basic treatment processes it is easy to see how
and why the processes are applied in the various alternative technologies
discussed later.
BASIC CONSTITUENTS OF WASTEWATER
Biochemical oxygen demand
One of the most commonly measured constituents of wastewater is the
biochemical oxygen demand, or BOD. Wastewater is composed of a variety
of inorganic and organic substances. Organic substances refer to molecules
that are based on carbon and include fecal matter as well as detergents,
soaps, fats, greases and food particles (especially where garbage grinders
are used). These large organic molecules are easily decomposed by bacteria
in the septic system. However, oxygen is required for this process of breaking
large molecules into smaller molecules and eventually into carbon dioxide
and water. The amount of oxygen required for this process is known as the
biochemical oxygen demand or BOD. The Five-day BOD, or BOD5,
is measured by the quantity of oxygen consumed by microorganisms during
a five-day period, and is the most common measure of the amount of biodegradable
organic material in, or strength of, sewage.
BOD has traditionally been used to measure of the strength of effluent
released from conventional sewage treatment plants to surface waters or
streams. This is because sewage high in BOD can deplete oxygen in receiving
waters, causing fish kills and ecosystem changes. Based on criteria for
surface water discharge, the secondary treatment standard for BOD has been
set at 30 mg BOD/L (i.e. 30 mg of O2 are consumed per liter of water over 5 days to break down the waste).
However, BOD content of sewage is also important for septic systems.
Sewage treatment in the septic tank is an anaerobic (without oxygen) process;
in fact, it is anaerobic because sewage entering the tank is so high in
BOD that any oxygen present in the sewage is rapidly consumed. Some BOD
is removed in the septic tank by anaerobic digestion and by solids which
settle to the bottom of the septic tank, but much of the BOD present in
sewage (especially detergents and oils) flows to the leaching field. Because
BOD serves as a food source for microbes, BOD supports the growth of the
microbial biomat which forms under the leaching field. This is both good
and bad. On the one hand, a healthy biomat is desired because it is capable
of removing many of the bacteria and viruses in the sewage so that they
do not pass to the groundwater. The bacteria in a healthy biomat also digest
most of the remaining BOD in the sewage. Too much BOD, however, can cause
excessive growth of bacteria in the biomat. If the BOD is so high that
all available oxygen is consumed (or if the leaching field is poorly aerated,
as can be the case in an unvented leaching field located under pavement
or deeply buried) the biomat can go anaerobic. This causes the desirable
bacteria and protozoan in the biomat to die, resulting in diminished treatment
of the sewage. Low oxygen in the biomat also encourages the growth of anaerobic
bacteria (bacteria which do not require oxygen for growth). Many anaerobic
bacteria produce a mucilaginous coating which can quickly clog the leaching
field. Thus, excess BOD in sewage can cause a leaching field to function
poorly and even to fail prematurely.
Many of the enhanced treatment technologies discussed later in this
document were designed specifically to reduce BOD in treated sewage. BOD
removal can be especially important where sewage effluent flows to a leaching
field in tight soils. Tight soils are usually composed of silts and clays
(particle size < 0.05 millimeter). These small soil particles are tightly
packed and the pore space between them is small. Reducing BOD means that
the sewage will support the growth of less bacteria and therefore the effluent
will be better able to infiltrate tight soils. Many enhanced treatment
technologies that remove BOD were designed specifically to enhance disposal
of effluent in tight silt or clay soils.
BOD is fairly easy to remove from sewage by providing a supply of oxygen
during the treatment process; the oxygen supports bacterial growth which
breaks down the organic BOD. Most enhanced treatment units described incorporate
some type of unit which actively oxygenates the sewage to reduce BOD. This
unit is often located between the septic tank and the leach field. Or,
it can be located within the septic tank in a specific area where oxygen
is supplied. Reduction of BOD is a relatively easy and efficient process,
and results in sewage of low BOD flowing to the leaching field. It is important
to note, however, that low BOD in sewage may result in a less effective
biomat forming under the leaching field.
It is also important to note that BOD serves as the food source for
the denitrifying bacteria which are needed in systems where bacterially-mediated
nitrogen removal takes place. In these situations BOD is desired, as the
nitrification/denitrification process cannot operate efficiently without
sufficient BOD to support the growth of the bacteria which accomplish the
process.
Total suspended solids
Domestic wastewater usually contains large quantities of suspended solids
that are organic and inorganic in nature. These solids are measured as
Total Suspended Solids or TSS and are expressed as mg TSS/ liter
of water. This suspended material is objectionable primarily because it
can be carried with the wastewater to the leachfield. Because most suspended
solids are small particles, they have the ability to clog the small pore
spaces between soil grains in the leaching facility. There are several
ways to reduce TSS in wastewater. The simplest is the use of a septic tank
effluent filter, such as the Zabel filter (several other brands are available).
This type of filter fits on the outlet tee of the septic tank. It is made
of PVC with various size slots fitted inside one another. The filter prevents
passage of floating matter out of the septic tank and, as effluent filters
through the slots, fine particles are also caught. Many types of alternative
systems are also able to reduce TSS, usually by the use of settling compartments
and/or filters using sand or other media.
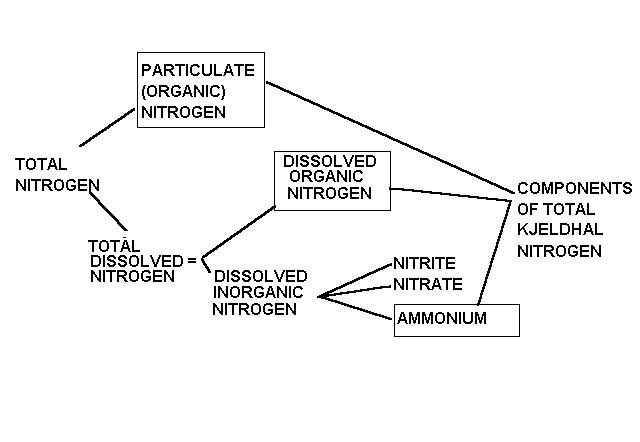
Total nitrogen
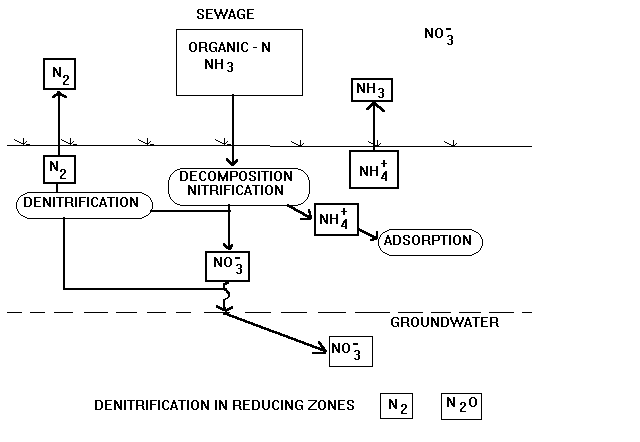
Nitrogen is present in many forms in the septic system. Most nitrogen
excreted by humans is in the form of organic nitrogen (dead cell material,
proteins, amino acids) and urea. After entering the septic tank, this organic
nitrogen is broken down fairly rapidly and completely to ammonia, NH3,
by microorganisms in the septic tank. Ammonia is the primary form of nitrogen
leaving the septic tank. In the presence of oxygen, bacteria will break
ammonia down to nitrate, NO3. In a conventional septic system
with a well aerated leaching facility, it is likely that most ammonia is
broken down to nitrate beneath the leaching field.
Nitrate can have serious health effects when it enters drinking water
wells and is consumed. Nitrate and other forms of nitrogen can also have
deleterious effects on the environment, especially in coastal areas where
excess nitrogen stimulates the process known as eutrophication. For this
reason, many alternative technologies have been designed to remove total
nitrogen from wastewater. These technologies use bacteria to convert ammonia
and nitrate to gaseous nitrogen, N2. In this form nitrogen is
inert and is released to the air.
Biological conversion of ammonia to nitrogen gas is a two step process.
Ammonia must first be oxidized to nitrate; nitrate is then reduced to nitrogen
gas. These reactions require different environments and are often carried
out in separate areas in the wastewater treatment system.
The first step in the process, conversion of ammonia to nitrite and
then to nitrate, is called nitrification (NH3  NO2
NO3). The process is summarized in the following equations: NO2
NO3). The process is summarized in the following equations:
It is important to note that this process requires and consumes oxygen.
This contributes to the BOD or biochemical oxygen demand of the sewage.
The process is mediated by the bacteria Nitrosomonas and Nitrobacter
which require an aerobic (presence of oxygen) environment for growth and
metabolism of nitrogen. Thus, the nitrification process must proceed
under aerobic conditions.
The second step of the process, the conversion of nitrate to nitrogen
gas, is referred to as denitrification. This process can be summarized
as:
This process is also mediated by bacteria. For the reduction of nitrate
to nitrogen gas to occur, the dissolved oxygen level must be at or near
zero; the denitrification process must proceed under anaerobic conditions.
The bacteria also require a carbon food source for energy and conversion
of nitrogen. The bacteria metabolize the carbonaceous material or BOD in
the wastewater as this food source, metabolizing it to carbon dioxide.
This in turn reduces the BOD of the sewage, which is desirable. However,
if the sewage is already low in BOD, the carbon food source will be insufficient
for bacterial growth and denitrification will not proceed efficiently.
Clearly, any wastewater treatment unit that is going to remove nitrogen
by the nitrification/denitrification process must be designed to provide
both aerobic and anaerobic areas so that both nitrification and denitrification
can proceed. As you look at the nitrogen removal technologies discussed
later in this document, you will see how various designs have attempted
to solve this problem in some unique and interesting ways.
Phosphorus
Phosphorus is a constituent of human wastewater, averaging around 10
mg/liter in most cases. The principal forms are organically bound phosphorus,
polyphosphates, and orthophosphates. Organically bound phosphorus originates
from body and food waste and, upon biological decomposition of these solids,
is converted to orthophosphates. Polyphosphates are used in synthetic detergents,
and used to contribute as much as one-half of the total phosphates in wastewater.
Several states have banned the sale of phosphate-containing clothes washing
detergent, so phosphorus levels in household wastewater have been reduced
significantly from previous levels. Virginia and fifteen other states banned phosphate-containing
dish detergent starting in 2010. Most household phosphate inputs now
come from human waste and automatic dishwasher detergent. Polyphosphates
can be hydrolyzed to orthophosphates. Thus, the principal form of phosphorus
in wastewater is assumed to be orthophosphates, although the other forms
may exist. Orthophosphates consist of the negative ions PO43-,
HPO42-, and H2PO4-.
These may form chemical combinations with cations (positively charged ions).
It is unknown how much phosphorus is removed in a conventional septic
system. Some phosphorus may be taken up by the microorganisms in the septic
system and converted to biomass (of course, when these microorganisms die
the phosphorus is re-released, so there really is no net loss of phosphorus
by this mechanism). Any phosphorus which is removed in the septic system
probably is removed under the leaching facility by chemical precipitation.
At slightly acidic pH, orthophosphates combine with tri-valent iron or aluminum
cations to form the insoluble precipitates FePO4 and AlPO4.
Domestic wastewater usually contains only trace amounts of iron and
aluminum. However, sandy soils frequently contains significant
amounts of iron bound to the surface of sand particles. It is likely that
this iron binds with phosphorus and causes some removal of total phosphorus
below the leaching facility.
One caveat must need be added here. If the soil below the leaching facility
becomes anaerobic, iron may become chemically reduced (changed to the Fe2+
form), which is soluble and able to travel in groundwater. In this case,
the iron phosphate compounds may breakdown and phosphorus may also become
soluble. Anaerobic conditions under the leaching facility can occur when
the leaching facility is not well aerated, when there is a small vertical
separation to groundwater, or when BOD in the sewage is so high that all
oxygen present is depleted to oxidize BOD. The best method for maximizing phosphorus removal is probably to locate
the leaching facility well above groundwater (>5 feet vertical separation)
thereby providing a well-aerated area under the leaching field. To date,
no alternative on-site technologies are capable of significant phosphorus
removal. However, many are trying to achieve this goal and it is likely
that within the next few years we may begin to see some technologies that
are successful at phosphorus removal.
BASICS OF SEWAGE TREATMENT
The treatment of sewage is largely a biochemical operation, where chemical
transformations of the sewage are carried out by living microorganisms.
Different environments favor the growth of different populations of microorganisms
and this in turn affects the efficiency, end products, and completeness
of treatment of the sewage. Sewage treatment systems, whether they are
standard septic systems or more advanced treatment technologies, attempt
to create specific biochemical environments to control the sewage treatment
process.
Three basic types of biochemical transformations occur as sewage is
treated. The first is the removal of soluble organic matter. This is composed
of dissolved carbon compounds such as detergents, greases, and body wastes,
which make up much of the BOD content of the sewage. The second is the
digestion and stabilization of insoluble organic matter. These are the
sewage solids, such as body wastes and food particles, which make up the
remainder of the BOD. The third is the transformation of soluble inorganic
matter such as nitrogen and phosphorus.
The two major biochemical environments in which sewage treatment is
carried out are termed aerobic and anaerobic environments.
An aerobic environment is one in which dissolved oxygen is available in
sufficient quantity that the growth and respiration of microorganisms is
not limited by lack of oxygen. An anaerobic environment is one in which
dissolved oxygen is either not present or its concentration is low enough
to limit aerobic metabolism. The biochemical environment has a profound
effect upon the ecology of the microbial population which treats the sewage.
Aerobic conditions tend to support entire food chains from bacteria up
to rotifers and protozoans. These microbes beak down organic matter using
many metabolic pathways based on aerobic respiration with carbon dioxide
as the main end product. Anaerobic conditions favor the growth of primarily
bacterial populations and produce a different variety of end products,
discussed below.
Anaerobic Digestion of Sewage
Solids in sewage contain large amounts of readily available organic
material that would produce a rapid growth of microorganisms if treated
aerobically. Anaerobic decomposition is able to degrade this organic material
while producing much less (approximately one-tenth) biomass than an aerobic
treatment process. The principal function of anaerobic digestion is to
stabilize insoluble organic matter and to convert as much of these solids
as possible to end products such as liquids and gases (including methane)
while producing as little residual biomass as possible. It is for this
reason that sewage treatment in a conventional septic tank is designed
to be an anaerobic process. Organic matter treated anaerobically is not
broken down to carbon dioxide; final end products are low molecular weight
acids and alcohols. These may be further converted anaerobically to methane
or, if sent to an environment (such as the leaching field) where aerobic
bacteria are present, further broken down to carbon dioxide. Anaerobic
digestion of organic matter is also a much slower process than aerobic
digestion of organics and where rapid digestion of organic matter is needed
an aerobic treatment process must be used.
As discussed above, an anaerobic environment is also necessary for
denitrification,
as the bacteria which carry out this process require anaerobic conditions
to reduce nitrate to nitrogen gas. Many nitrogen-removal technologies are
designed to provide an anaerobic treatment chamber as part of the treatment
process.
Aerobic Treatment of Sewage
As the name implies, this process utilizes aerobic bacteria to break
down sewage. The principal advantage of aerobic sewage treatment is its
ability to rapidly and completely digest sewage, reducing BOD to low levels.
Most of the alternative treatment technologies discussed in this document
utilize some form of aerobic treatment of sewage. This process is used
primarily to reduce BOD and, in systems that remove nitrogen, to nitrify
the waste so that it can later be denitrified. Because the BOD in raw sewage
is usually high, and available oxygen is rapidly consumed by the sewage,
most aerobic treatment units are designed to supply supplemental oxygen
to the sewage to keep the treatment process aerobic. Some units, such as
the JET Aerobic system, use extended aeration to more completely
digest the sewage solids. Most aerobic treatment units provide some type
of artificial medium as a surface on which the sewage- digesting bacteria
can grow. A variety of basic designs can be used for this purpose.
Attached culture systems are designed so that wastewater flows
over microbial films attached to surfaces in the treatment unit. The surface
area for growth of the biofilm is increased by placing some type of artificial
media, such as foam cubes or various convoluted plastic shapes with high
surface area, in the treatment chamber. This artificial media may sit in
the treatment chamber with the effluent circulating through it, usually
with supplemental air supplied so that treatment remains aerobic. This
is the principal used by the JET Aerobic and FAST
systems. Or, the media may be located outside the treatment chamber and
wastewater is passed over the biofilm in intermittent doses. These designs
are known as trickle filters and are one of the most common types
of on-site treatment unit using attached cultures. Some technologies which
employ trickle filters, and which are discussed in more detail later, include
the Bioclere, Orenco trickle filter, and the Waterloo biofilter.
Intermittent and recirculating sand filters, while located in separate
chambers, can also be considered a form of trickle filter where sand is
used as the media for bacterial growth. Because attached culture systems
are generally aerobic, a complex community of microorganisms, including
aerobic bacteria, fungi, protozoa, and rotifers, develops. These systems
are capable of efficient removal of BOD. Being aerobic they will support
the growth of nitrifying bacteria and can be used to nitrify wastewater,
the first step in nitrogen removal.
Other aerobic systems utilize suspended culture of microorganisms
to aerobically treat the sewage. This type of treatment assumes that a
resident population of bacteria are present in the solids and sludge in
the treatment unit; vigorous mixing of the sewage in the treatment compartment
causes these bacteria to stay in suspension where they can aerobically
digest the sewage. This principle is used by the Cromaglass and
Amphidrome units as part of part of the batch reactor treatment
process. It is also used in many large municipal sewage treatment plants.
The activated sludge process is similar to suspended culture
in that it also utilizes the resident population of bacteria in the solids
and sludge in the treatment unit, again, usually by mixing of the sewage
so that the bacteria are kept in suspension. In the activated sludge process,
however, there are usually periods where mixing ceases, and the solids
are allowed to settle. It is then assumed that the sludge will become anaerobic
and the anaerobic bacteria in the sludge will denitrify the waste. This
is the principle used by batch reactors. As the name implies, batch
reactors treat sewage in batches. A batch of sewage is allowed to settle
so that solids are removed; the batch of sewage is then aerated and mixed
and then allowed to settle for a period of anaerobic treatment (this process
may be repeated several times on the same batch). When treatment is complete,
the finished batch of sewage is pumped out and the next batch enters the
unit to begin treatment. The Cromaglass and Amphidrome systems
are examples of batch reactors.
References
Grady, C.P. Leslie and Henry C. Lim, 1980. Biological Wastewater
Treatment. Marcel Decker, Inc., N.Y
Peavey, Howard S., Donald R. Rowe, and George Tchobanoglous, 1985. Environmental
Engineering, McGraw Hill Inc., N.Y.
 | 


